Full 2019 US revenues for Humira were 149 billion up 86 from 2018. Im Jahr 2020 setzte das Unternehmen mit dem monoklonalen Antikörper Humira.
HUMIRA can be used alone with methotrexate or with certain other medicines.
Humira black market. Prices of for two kits of Humira Pen 40 mg in the US. Metrics for Humira in the 10MM Ulcerative Colitis UC markets during the forecast period from 20122022. Use is generally only recommended in people who have not responded to other treatments.
On averagecost some 4500 US. For more information talk to your health care provider. Raymond rates AbbVie Overweight.
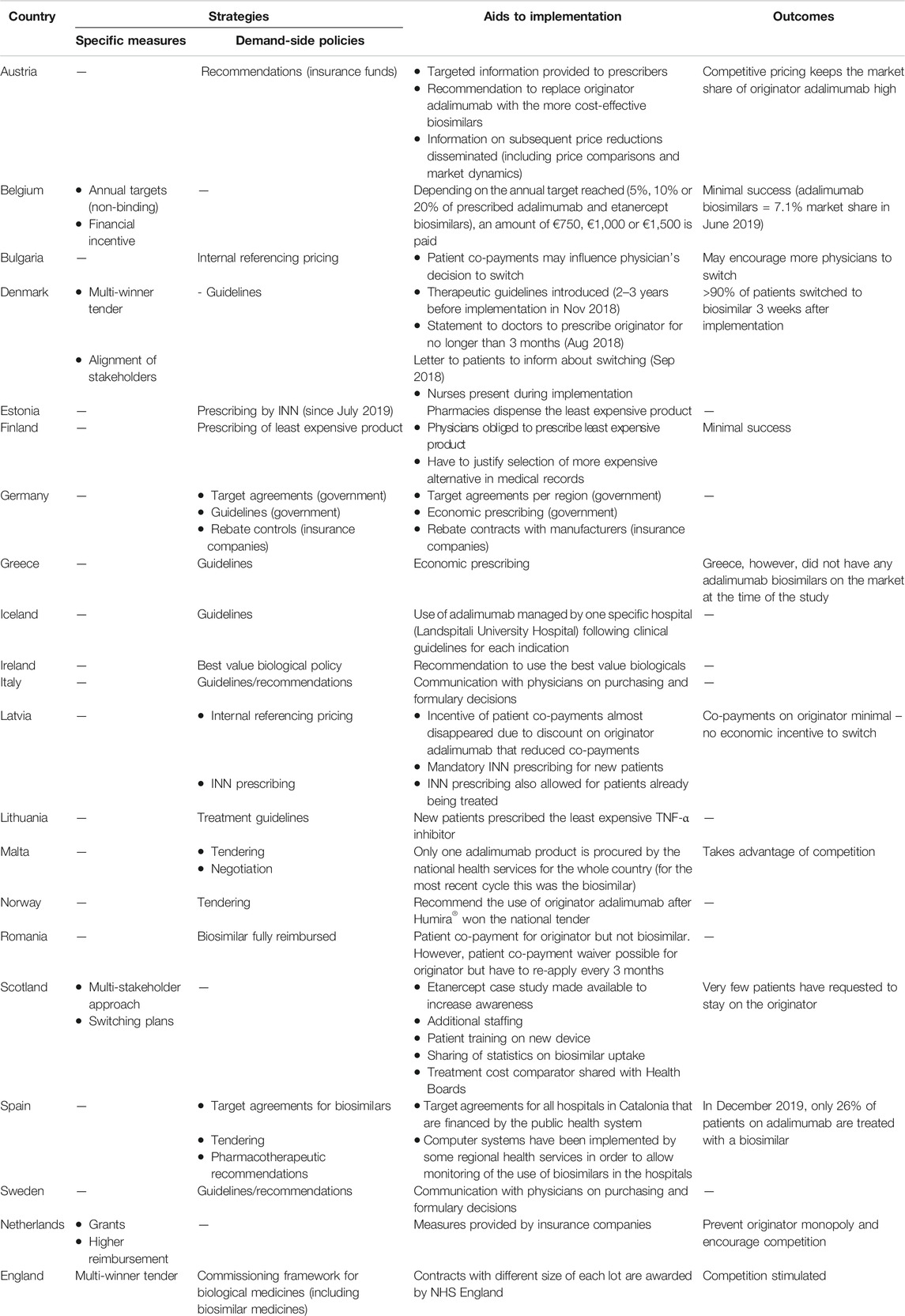
A more significant threat to Humiras market share may be posed by newly approved therapies such a JAK inhibitors - shown to be superiority over Humira in the treatment of RA during clinical trials. Das Biotech- und Pharmaunternehmen AbbVie wurde 2013 als Abspaltung von Abbott Laboratories gegründet. But 2019 also saw heavy biosimilar competition in Europe eroding market share for Humira which outside.
Humira is used to treat many inflammatory conditions in adults such as ulcerative colitis Rheumatoid Arthritis psoriatic arthritis Ankylosing Spondylitis plaque psoriasis and a skin condition called. It also features cancers. The cost of Humira which is injected via syringe was more than 72000 a year on prescription drug websites this week and is not.
Raymond said that he expects sales of Humira of 192 billion world-wide in 2020 more than the FactSet consensus of 191 billion. To reduce the signs and symptoms of. HUMIRA is a tumor necrosis factor TNF blocker medicine that can lower the ability of your immune system to fight infections.
Ads for several including Humira and Cosentyx are fixtures on television promising improved. These serious infections include tuberculosis TB and infections caused by viruses. At least 9 biosimilar adalimumab products are lined up to enter the US market in 2023 so these are important final years of dominance for Humira which entered the US market in 2003.
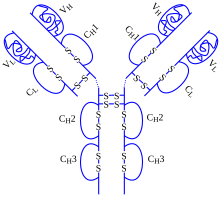
Adalimumab sold under the brand name Humira among others is a medication used to treat rheumatoid arthritis psoriatic arthritis ankylosing spondylitis Crohns disease ulcerative colitis psoriasis hidradenitis suppurativa uveitis and juvenile idiopathic arthritis. Humira was among a handful of drugs with the highest jumps. Global Humira Market Size 2020 to 2025 Globally the size of the Humira market is expected to grow at a vibrant CAGR between 2020 to 2025.
Serious infections have happened in people taking HUMIRA. TNF alpha-Inhibitor humaner monoklonaler Antikörper. Tätig ist AbbVie unter anderem in den Bereichen Immunologie Onkologie und Virologie.
A safety signal with Pfizers Xeljanz in the same drug class gives pause as to the extent of the market traction of JAK inhibitors including Rinvoq. 10 LIM CHF 66180. Dies ist keine vollständige Liste der mit Humira.
HUMIRA Inj Lös 20 mg02ml Adalimumab. Humira can be also be used for diseases such as Plaque psoriasis and Crohns disease. HUMIRA may prevent further damage to.
Moderate to severe rheumatoid arthritis RA in adults. You should not start taking HUMIRA if you have any kind of infection unless your doctor says it is okay. It is administered by.
Dollars as of 2017. Humira was initially approved in the United States and is now available in more than 60 countries. Key Metrics in the 10MM UC Markets 20122022 2012 Market Sales US 60276m 5EU 53969m Japan 1646m Canada 8056m China and India NA Total 124bn Key Events 20122022 Level of Impact.
With AbbVies originator brand bringing in more than 18 billion in 2017 sales 44 billion of which came from European revenues the arrival of competition from Humira biosimilars. A black box warning in Humiras label highlights the risk of serious infections leading to hospitalization or death including TB bacterial sepsis invasive fungal infections and infections due to opportunistic pathogens. 2 Fertigspritzen 02 ml.
Humira has a black box warning because it can increase the risk of serious infections including tuberculosis TB and pneumonia This is because Humira can weaken your immune system and make it. Humira wird zur Behandlung von rheumatoider Arthritis psoriatischer Arthritis juveniler idiopathischer Arthritis Plaque-Psoriasis und Morbus Crohn eingesetzt. This is the most important information to know about HUMIRA.
In the past 20 years the drugs which can cost some 40000 a year have flooded the market. Die Statistik zeigt den Umsatz des Pharmaunternehmens AbbVie mit dem Arzneimittel Humira in den Jahren 2011 bis 2020. AbbVies strategy to blunt headwinds from the entry of Humira adalimumab biosimilars in 2023 with its JAK inhibitor Rinvoq upadacitinib is constrained by recent news experts said.
He raised his price target to. HUMIRA is a prescription medicine used. Einige der häufigsten Nebenwirkungen von Humira sind Kopfschmerzen Hautausschlag Nebenhöhlenentzündung Übelkeit Harnwegsinfektion hoher Cholesterinspiegel Bauchschmerzen Rückenschmerzen und Bluthochdruck.
Humira Market 2019-2024 Humira adalimumab is a tumor necrosis factor TNF blocker that reduces the effects of a substance in the body that can cause inflammation.