LLY today jointly announced that the US. Lilly unites caring with discovery to create medicines that make life better for people around the world.
 Eli Lilly Compelling Risk Reward Nyse Lly Seeking Alpha
Eli Lilly Compelling Risk Reward Nyse Lly Seeking Alpha
The drugmakers revamped focus will also feature a more aggressive approach to business development that will target early-stage pipeline additions.
Eli lilly oncology pipeline. ALIMTA CYRAMZA ERBITUX Retevmo and Verzenio are trademarks owned by or licensed to Eli Lilly and Company its subsidiaries or affiliates. Eli Lilly LLY has outperformed the SP index and its competitors during the past 5 years LLY has a broad product portfolio a robust pipeline that is. An experimental cancer drug being tested by Eli Lilly did not extend survival versus a common chemotherapy regimen in patients with metastatic pancreatic cancer the latest in a series of clinical setbacks for drugmaker efforts to develop.
At Lilly Oncology we are dedicated to developing and delivering innovative new medicines that will make a meaningful difference to cancer patients. 16 2019 Ned Pagliarulo Lead Editor. Lillys research team is leveraging precision medicine and immuno-oncology to bring forth new medicines as fast as possible to help people living with cancer fight their disease.
A pair of Eli Lillys cancer prospects that made it through a pipeline cull in 2017 werent so lucky come 2019. SAN FRANCISCO CA USA and SUZHOU China I May 18 2021 I Innovent Biologics Inc. At Lilly Oncology some of us have been touched by cancer one way or anothereither as a patient or as a caregiver relative or friend of a patient.
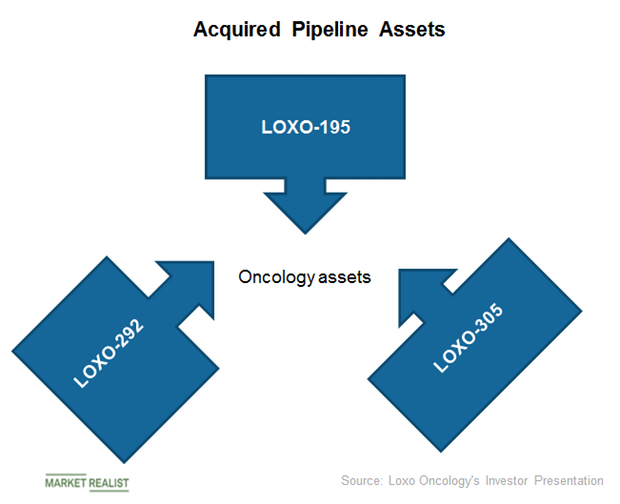
Loxo Oncologys approved oncology asset According to Eli Lillys investor presentation the acquisition will add VITRAKVI larotrectinib which was approved via an accelerated approval pathway by. Global Aurora-A Kinase Inhibitors Pipeline Insight Report 2021. The Big Pharma revealed in its first-quarter earnings presentation that it is dumping.
TYVYT sintilimab injection Innovent This press release contains forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995 about Lilly s oncology portfolio and pipeline. In two firsts Eli Lilly has won its first approval for a pipeline drug coming out of the 8 billion acquisition of Loxo Oncologyand its the. Transcenta is developing a pipeline of nine innovative molecules for the treatment of cancer bone and kidney disorders.
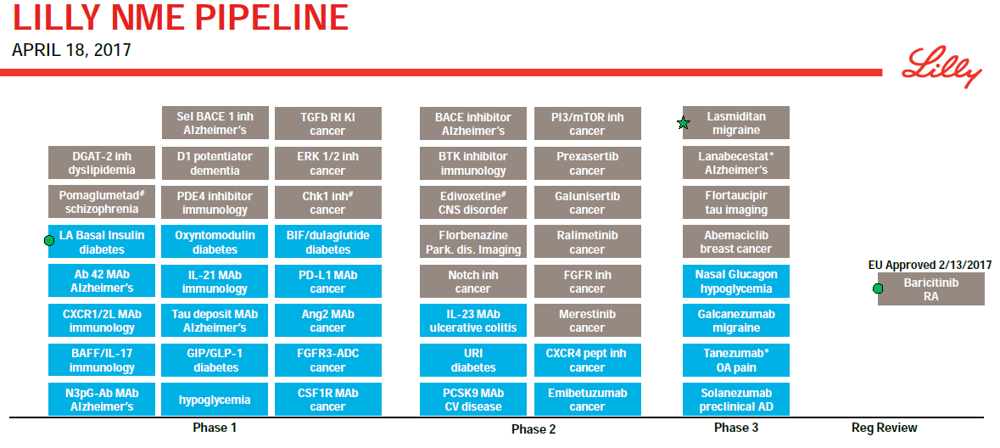
TransThera Biosciences Eli Lilly and Co Takeda Oncology Wigen Biomedicine TT-00420 LY-3295668 Alisertib WJ-05129. Eli Lilly Co. LLY will present data from several molecules that make up its clinical oncology pipeline during the 48th Annual Meeting of the American Society of Clinical Oncology ASCO held in Chicago Ill.
About Eli Lilly and Company External Innovation. Cancer disrupts life and changes its course and were determined to provide support beyond treatment to you and your loved ones to help ease the difficult times and celebrate the good ones. In announcing a new RD strategy Lilly signaled it intends to compete in oncology and build on a portfolio that currently lags behind leaders like Bristol-Myers Squibb Merck and Roche.
Lilly cancer drug pipeline hit by trial setback Published Oct. Two weeks into the Josh Bilenker era at Eli Lilly the legacy pharma was excited to spotlight three early-stage priority oncology programs as they issued their 2020 guidance. 01801 and Eli Lilly and Company NYSE.
Lilly cuts a trio of pipeline assets including BTK inhibitor diabetes drug Eli Lilly returned the rights to the BTK inhibitor being developed for rheumatoid arthritis back to. Lilly has a robust oncology pipeline that includes both small molecules and monoclonal antibodies which are being studied to treat a wide range of cancers including breast colorectal liver and non-small cell lung. INDIANAPOLIS May 14 2012 PRNewswire -- Eli Lilly and Company NYSE.
Food and Drug Administration FDA accepted for review a Biologics License Application BLA for sintilimab injection in combination with pemetrexed and platinum.