Aetion delivers real-world evidence RWE and outcomes-based analytics solutions to life sciences companies payers and at-risk providers. Aetion a four-year-old health care startup that aims to bridge the gap between providers payers and biopharmaceutical companies with a novel analytics solution today announced that it has.
 Real World Evidence Platform Rwe Solution Aetion
Real World Evidence Platform Rwe Solution Aetion
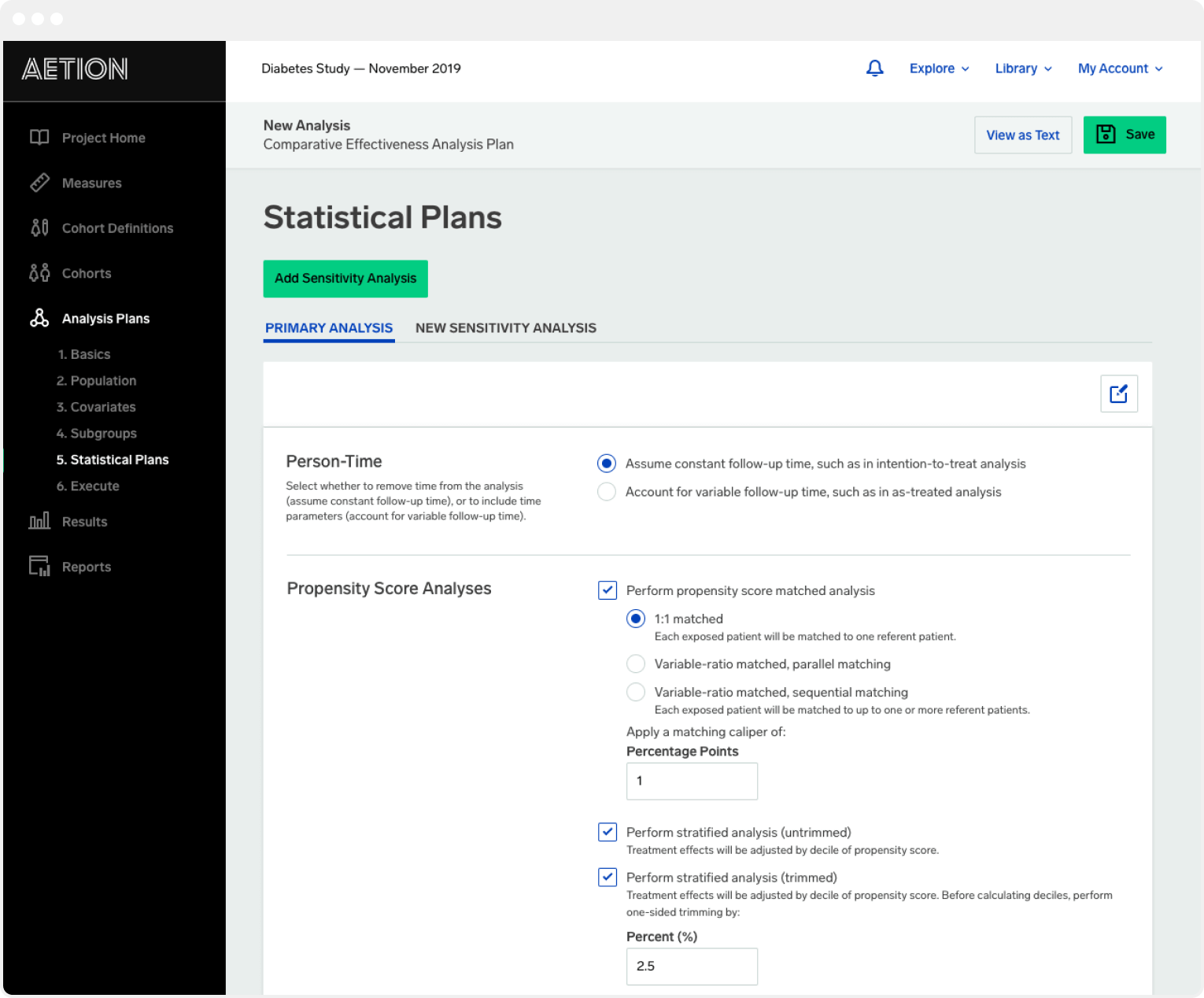
To make this process more efficient its helpful to use a platform that is data agnostic such as the Aetion Evidence Platform AEP.

Aetion evidence platform. Its validated rapid-cycle analytics allows users to significantly reduce time to evidence and improve patient outcomes. ICER adopts Aetions Real-World Evidence Platform advancing standards for the use of transparent replicable real-world evidence in health technology assessment NEW YORK and BOSTON February 24 2020 Today the Institute for Clinical and Economic Review ICER announced it has selected Aetion as a preferred partner and platform to generate decision-grade real-world. Building on decades of experience in epidemiology and health outcomes research our founders joined forces with out-of-industry technologists to develop the Aetion Evidence Platform AEPscientifically validated software for performing rapid analyses to generate real-world evidence at scale.
Food and Drug Administration now uses the Aetion Evidence Platform AEP as it develops more modern drug approval and safety. Aetion delivers real-world evidence news analysis and insights to accelerate the adoption of RWE throughout the pharmaceutical and drug research industry. Filed in March 5 2019 the AETION EVIDENCE PLATFORM covers Computer software consultancy.
Aetion became the first RWE company to establish an FDA COVID-19 research collaboration agreement and was selected by ICER as its preferred RWE partner and platform. Its main product the Aetion Evidence Platform is able to analyze data from real-world evidence in order to help validate the safety effectiveness and value of certain treatments or procedures. What sets the Aetion Evidence Platform apart is the deep scientific expertise that has informed the technology along every step of the way.
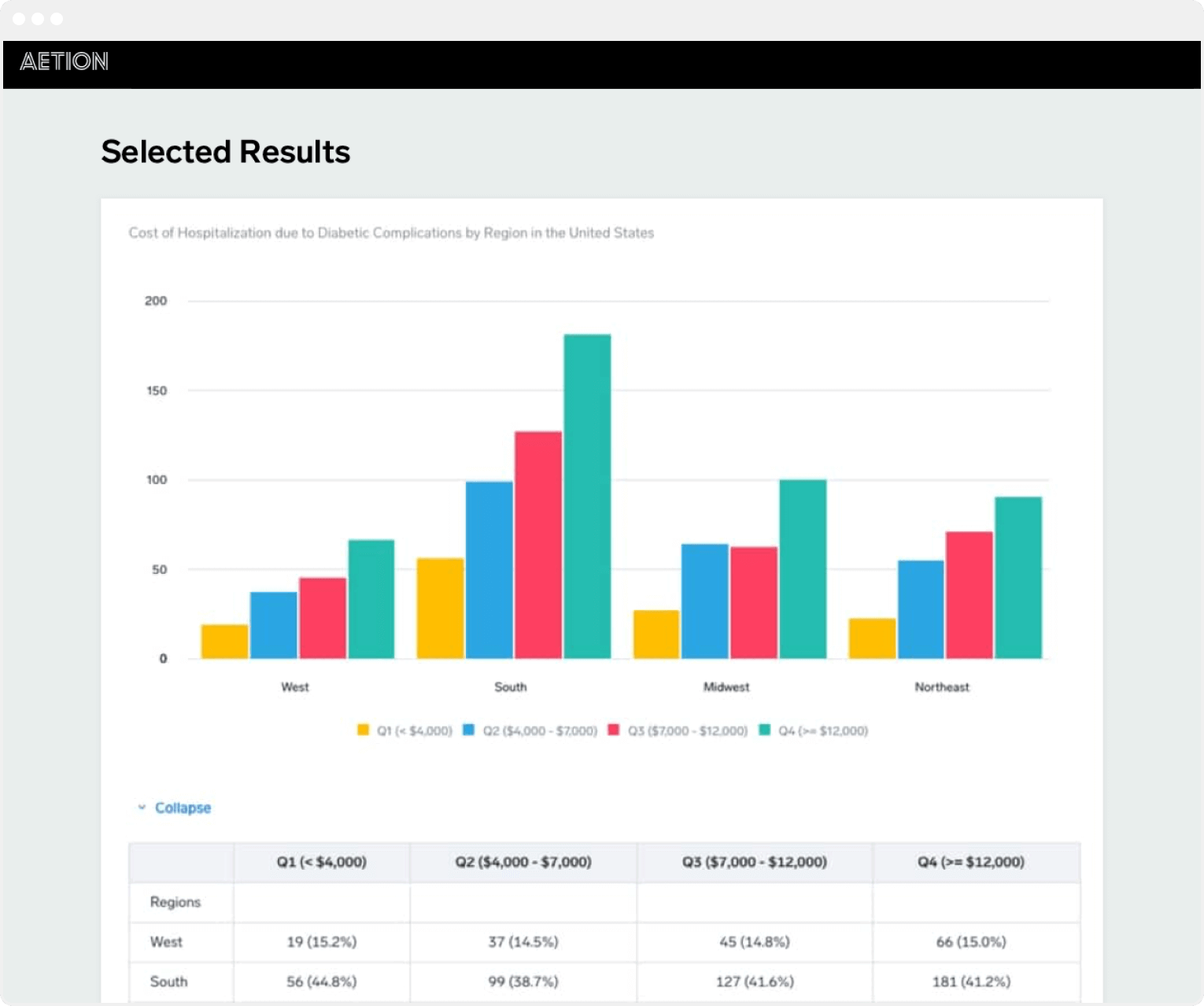
The Aetion Evidence Platform uses the everyday clinical and financial interactions of the health care system to unlock essential evidence about the effectiveness and value of treatments. In a post-COVID era we have an imperative to use rapid rigorous evidence to inform health care decisions said Aetion CEO Carolyn Magill. The Aetion Evidence Platform analyzes data from the real world to produce transparent rapid and scientifically validated answers on costs and outcomes.
Aetion headquartered in New York with offices in Boston Los Angeles and Paris is a rapidly growing healthcare technology startup company showing groundbreaking results from the use of real world evidence RWE. The technology is specifically built to engender trust and alignment across stakeholders bringing biopharma together. Its use cases include RD optimization data review for regulatory approval and treatment effectiveness for certain populations.
Aetion has recently partnered with the FDA to use their Evidence Platform to better explore the history of the Covid-19 and integrate real world evidence for best. Construct the control arm Once we have the data in a workable format and accessible within the platform we focus. Platform as a service PAAS featuring computer software platforms for collecting storing indexing analyzing updating managing sharing and reporting healthcare data population data.
With its patented rapid-cycle analytics technology the Aetion Evidence Platform utilizes the everyday clinical and financial interactions of the healthcare system to unlock essential evidence about the. In addition the US. Consider how Aetion Evidence Platform can help you generate decision-ready RWE The time to prepare for FDAs coming RWE guidance is now.
Real World Evidence Platform Rwe Solution Aetion
 Real World Evidence Solutions Rwe Analytics Aetion
Real World Evidence Solutions Rwe Analytics Aetion
 Eric St Onge Projects Aetion Evidence Platform
Eric St Onge Projects Aetion Evidence Platform
 Aetion Announces 36 Million In Series B
Aetion Announces 36 Million In Series B
 Real World Evidence Platform Rwe Solution Aetion
Real World Evidence Platform Rwe Solution Aetion
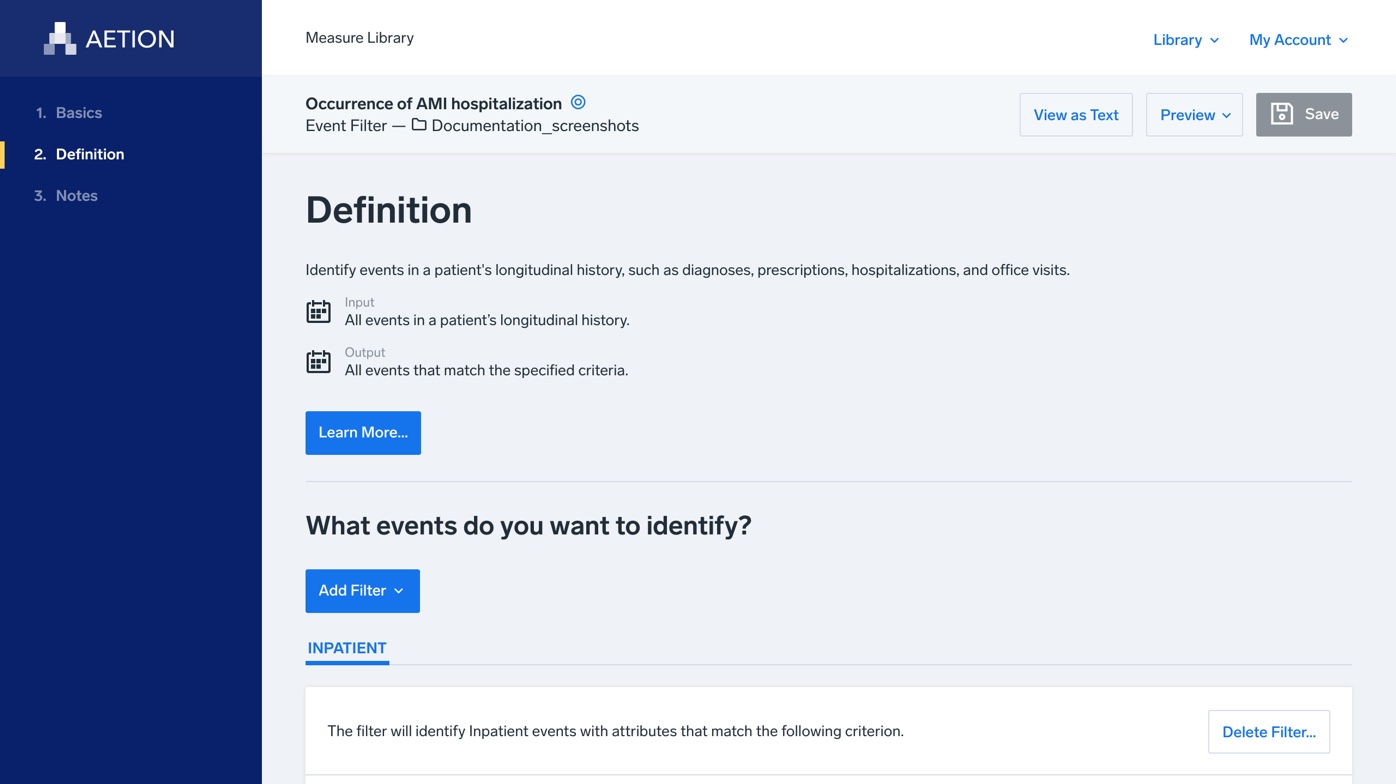
Building A Rule Based Validation Framework Rvf For Real World Healthcare Data By Feng Zhang Aetion Technology Medium
 Real World Evidence Platform Rwe Solution Aetion
Real World Evidence Platform Rwe Solution Aetion
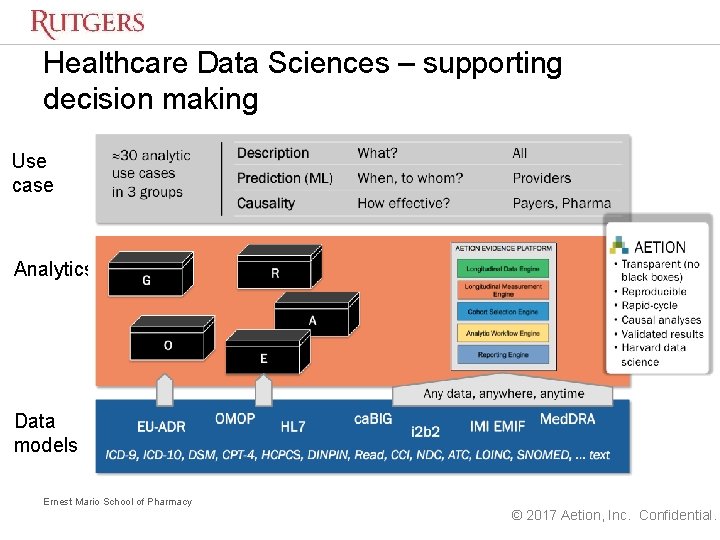 Enabling Essential Evidence For Healthcare Sebastian Schneeweiss Md
Enabling Essential Evidence For Healthcare Sebastian Schneeweiss Md
 Infographic What Role Does Rwe Play In Fda Approvals
Infographic What Role Does Rwe Play In Fda Approvals
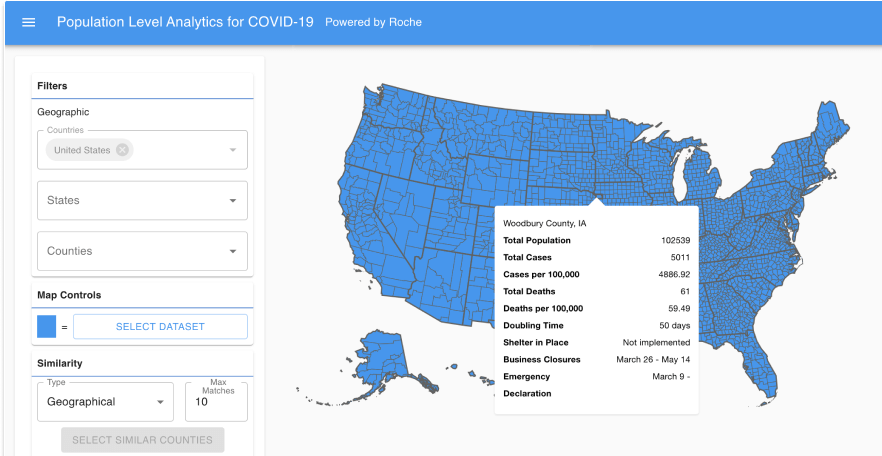
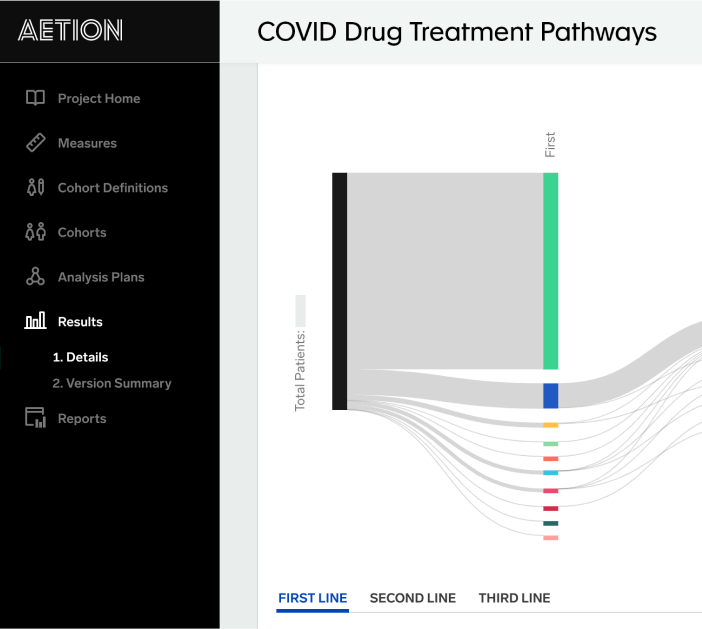 Covid 19 Real World Evidence System Aetion
Covid 19 Real World Evidence System Aetion
 Real World Evidence Platform Developer Aetion Nabs Amgen Backed 36m Series B Fiercebiotech
Real World Evidence Platform Developer Aetion Nabs Amgen Backed 36m Series B Fiercebiotech
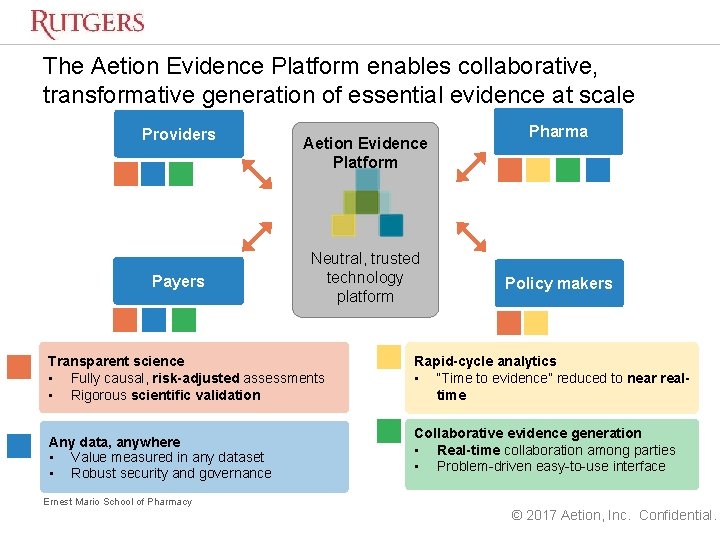 Enabling Essential Evidence For Healthcare Sebastian Schneeweiss Md
Enabling Essential Evidence For Healthcare Sebastian Schneeweiss Md



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.