Final results from a pilot study with an implantable loop recorder to determine the etiology of syncope in patients with negative noninvasive and invasive testing. Reveal LINQ Insertable Cardiac Monitoring ICM.
 Medtronic Receives Fda Clearance For Reveal Linq Insertable Cardiac Monitor With Trurhythm Detection Daic
Medtronic Receives Fda Clearance For Reveal Linq Insertable Cardiac Monitor With Trurhythm Detection Daic
Youve had your Reveal LINQ ICM System for a few weeks or more.
Medtronic loop recorder. The Medtronic Reveal LINQ Model LNQ11 Insertable Cardiac Monitor ICM is an MR Conditional device and as such is designed to allow patients to be safely scanned by a magnetic resonance imaging MRI machine. Is it still safe to defibrillate with aed Answered by a verified doctor. Direct patient line for fast service 1-866-470-7709.
Medtronic declara que este producto cumple los requisitos esenciales de la Directiva 19995CE sobre equipos radioeléctricos y equipos terminales de telecomunicación. External Loop Recorder Tilt Test EP Study Recurrent 12-lead ECG or 7-day Holter monitor. Can be performed in any standard procedure suite.
Only 800 551-5544 x41835 Toll free Monday - Friday 8 am to 5 pm Central Time. Long-term heart monitoring is designed for use over a period of months or even up to three years. Krahn AD Klein GJ Yee R Norris C.
Placement of an implantable loop recorder ILR. It allows for long-term heart rhythm monitoring. We hope you find these resources useful.
Medtronic Cardiac Diagnostics Monitoring is committed to providing you and your patients with a variety of patient education resources. What happens if someone goes into cardiac arrest and they have a linq reveal loop recorder implant. This is a question for the manufacturer and their enginee.
Medtronic Stay Connected Service A specialized patient service for monitor troubleshooting connectivity issues and other questions. Information for Patients Patient Services US. By Model Number such as 9528 SPS00876 3875-45 305U219 ENSP30030W Model Number.
By Product Name such as Revo MRI SynchroMed Endeavor Mosaic Delta NIM Flex. Medtronic is committed to providing quality customer service. Is typically inserted in the left parasternal region.
A cardiac loop recorder is a small insertable device that continuously monitors your hearts electrical activity and records it either automatically or when you use a. If you need a response during the same business day call Medtronic at 800-633-8766 toll-free within the United States or 1 763 514 4000 worldwide. Para obtener más información póngase en contacto con Medtronic en los números de teléfono y las direcciones que se proporcionan en la contraportada de este manual.
The machine works as an electrocardiogram ECG continuously picking up electrical signal from your heart. Expand All Collapse All. Page 50 EuropeMiddle EastAfrica Medtronic International Trading Sàrl Manufacturer Route du Molliau 31 Medtronic Inc.
How Is The Cardiac Loop Recorder Implanted. He or she places the small device under your skin on your chest wall overlying the heart. LIVING WITH YOUR REVEAL LINQ ICM SYSTEM.
From this site you can view print or order technical manuals free of charge for many Medtronic products. BHRS Standards for insertion follow up and explant of implantable loop recorders ILRs by non-medical staff 2018. Prophylactic antibiotics are administered intravenously prior to.
Short video demonstrating how to troubleshoot the 3230 error code on the MyCareLink patient monitor. An implantable loop recorder can help answer questions about your heart that other heart-monitoring devices dont provide. 11 Di Odoardo LAF et al.
Preclinical testing has demonstrated that the Reveal LINQ device is safe for use in the MRI environment when used. Reveal LINQ Insertable Cardiac Monitoring System provides long-term monitoring for detection of irregular heartbeats. During a loop recorder implantation your heart healthcare provider cardiologist does a minor procedure.
However due to the number of online inquiries we receive we may not be able to respond within the same business day. It can capture information that a standard electrocardiogram ECG or EKG or Holter monitor misses because some heart rhythm abnormalities occur infrequently.
 Implantable Loop Recorder Insertable Cardiac Monitor And Upload Download Scientific Diagram
Implantable Loop Recorder Insertable Cardiac Monitor And Upload Download Scientific Diagram
 Medtronic Receives Fda Clearance For Reveal Linq Insertable Cardiac Monitor With Trurhythm Detection Hhm Global B2b Online Platform Magazine
Medtronic Receives Fda Clearance For Reveal Linq Insertable Cardiac Monitor With Trurhythm Detection Hhm Global B2b Online Platform Magazine
Cardiology Medtronic S New Reveal Linq Device Revolutionises Diagnosis
 Reveal Linq Monitor Oliver Segal Cardiologist
Reveal Linq Monitor Oliver Segal Cardiologist
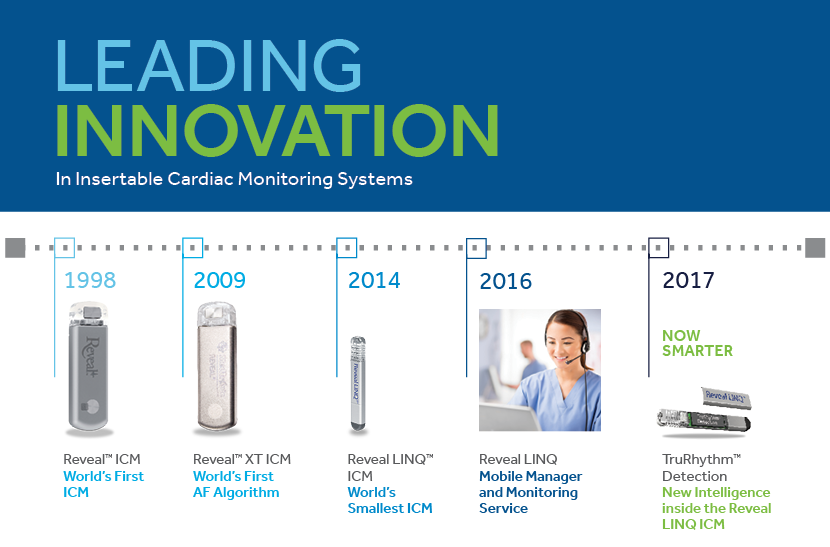 Medtronic Receives Fda Clearance For Reveal Linq Insertable Cardiac Monitor Icm With Trurhythm Detection Medical Design And Outsourcing
Medtronic Receives Fda Clearance For Reveal Linq Insertable Cardiac Monitor Icm With Trurhythm Detection Medical Design And Outsourcing
 Reveal Linq System Introduction For Patients Youtube
Reveal Linq System Introduction For Patients Youtube
 The Case Of The Migrating Loop Recorder Sciencedirect
The Case Of The Migrating Loop Recorder Sciencedirect
 Initial Real World Experience With A Novel Insertable Reveal Linq Medtronic Compared To The Conventional Reveal Xt Medtronic Implantable Loop Recorder At A Tertiary Care Center Points To Ponder International Journal Of
Initial Real World Experience With A Novel Insertable Reveal Linq Medtronic Compared To The Conventional Reveal Xt Medtronic Implantable Loop Recorder At A Tertiary Care Center Points To Ponder International Journal Of
 Part 2 Has My A Fib Returned 21 Day Results From My Medtronic Reveal Linq Loop Recorder Atrial Fibrillation Resources For Patients
Part 2 Has My A Fib Returned 21 Day Results From My Medtronic Reveal Linq Loop Recorder Atrial Fibrillation Resources For Patients
 Cardiac Monitors Reveal Linq Icm System Medtronic
Cardiac Monitors Reveal Linq Icm System Medtronic
 Medtronic S Tiny Heart Monitor Gets More Accurate Gains Fda Nod Fiercebiotech
Medtronic S Tiny Heart Monitor Gets More Accurate Gains Fda Nod Fiercebiotech
 Reveal Linq Cardiac Monitoring For Unexplained Syncope Patients Youtube
Reveal Linq Cardiac Monitoring For Unexplained Syncope Patients Youtube


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.