Mylans Unit 8 in Andhra Pradesh India was targeted as part of the fallout from a series of global recalls for sartan-based blood pressure. These drugs induce lamotrigine glucuronidation and increase clearance see Drug Interactions 7 Clinical Pharmacology 123.
 Mylan Recalls Drug For Heartburn Ulcers Over Cancer Concerns Fox Business
Mylan Recalls Drug For Heartburn Ulcers Over Cancer Concerns Fox Business
Has issued a voluntary nationwide recall of a batch of anti-anxiety medication sold under the brand name Xanax.
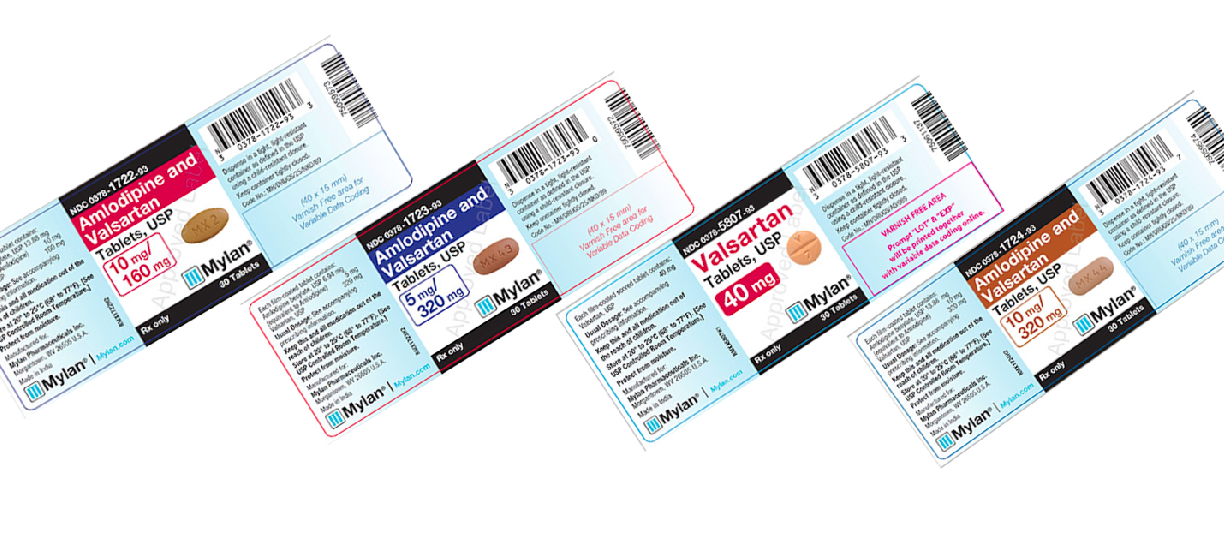
Mylan drug recall. Currently the recall affects only medications containing valsartan losartan or irbesartan. Is conducting a voluntary recall of the above referenced products manufactured by Mylan Pharmaceuticals Inc Morgantown WV utilizing Active Pharmaceutical Ingredient API supplied by Smruthi Organics Ltd a third party raw material manufacturer. Mylan Initiates Voluntary Nationwide Recall of 15 Lots of Valsartan Tablets USP Amlodipine and Valsartan Tablets USP and Valsartan and Hydrochlorothiazide Tablets USP Due to.
However not all lots of these medications are affected and being recalled. MORGANTOWN WVa Oct. Canonsburg-based pharmaceutical company Mylan produces Valsartan one of the drugs recalled in recent months.
Manufacturers are recalling medications containing amlodipine in combination with valsartan or losartan and medications containing hydrochlorothiazide HCTZ in combination with valsartan or losartan. Search ARB Recalls List Angiotensin II Receptor Blockers ARBs - A Message for Patients UPDATE - FDA warns Mylan for CGMP deviations. Mylan Pharmaceuticals is voluntarily recalling the lot of Alprazolam Tablets USP C-IV 05 mg.
Mylan Expands Its Voluntary Nationwide Recall of Valsartan Tablets USP Amlodipine and Valsartan Tablets USP and Valsartan and Hydrochlorothiazide Tablets USP to. December 5 2018 159 PM MoneyWatch Pharmaceutical firm Mylan is dramatically expanding a nationwide recall of some blood pressure medications after detecting trace amounts of a chemical linked. Mylan Pharmaceuticals has issued a voluntary nationwide recall of a batch of Xanax alprazolam tablets due to the potential presence of a foreign substance The announcement came after the company learned a batch of the popular prescription anti-anxiety medication may include a foreign material which could cause infection.
This lot is being recalled due to the potential presence of foreign substance. To see if your medication. Drugs Pharmaceuticals FDA Mylan Pharmaceuticals Inc.
The drug company is pulling. Valsartan tablets combination tablets with the drugs valsartan and amlodipine and combination tablets with. REASON Mylan Institutional Inc.
The generic drug company Mylan Pharmaceuticals recalled 104 lots of three medications. The FDA recall notice states that there is a small chance that consumers taking the affected Xanax could experience infection as a result. Mylan Hikma versions of injectable diuretic recommended for recall by Kentucky researchers Researchers are asking the FDA to issue a recall for two generic versions of acetazolamide.
The anti-anxiety medication is recalled due to the potential presence of foreign substance Mylan declined to provide additional information about the product or foreign substance. Is conducting a voluntary nationwide recall of one lot see table below of Alprazolam Tablets USP C-IV 05 mg to the consumeruser level. Other drugs which have similar effects include estrogen-containing oral contraceptives see Drug Interactions 7 Clinical Pharmacology 123.
25 2019 PRNewswire -- Mylan Pharmaceuticals Inc. The recall is due to potential contamination with a foreign substance. In a statement Mylan said it has taken immediate action to eliminate NDEA from the.
 Valsartan Recall Expanded To Include All Of One Company S Products
Valsartan Recall Expanded To Include All Of One Company S Products
 Recall Of Generic Version Of Xanax Is Announced By F D A The New York Times
Recall Of Generic Version Of Xanax Is Announced By F D A The New York Times
 Xanax Recalled Over Foreign Substance Concerns Fda Says
Xanax Recalled Over Foreign Substance Concerns Fda Says
 Partial Recall Of Anxiety Med Alprazolam Announced
Partial Recall Of Anxiety Med Alprazolam Announced
 Mylan Pharmaceuticals Initiates Voluntary Nationwide Recall Of One Lot Of Alprazolam Tablets Usp C Iv 0 5 Mg Due To The Potential Of Foreign Substance Fda
Mylan Pharmaceuticals Initiates Voluntary Nationwide Recall Of One Lot Of Alprazolam Tablets Usp C Iv 0 5 Mg Due To The Potential Of Foreign Substance Fda
 Recall Ndma Identified In Mylan S Nizatidine Capsules Mpr
Recall Ndma Identified In Mylan S Nizatidine Capsules Mpr
 Mylan Recalls One Lot Of Daptomycin For Injection Mpr
Mylan Recalls One Lot Of Daptomycin For Injection Mpr
 Medication Recall Alert Mylan Pharmaceuticals Recalls Xanax Drug And Device Watch
Medication Recall Alert Mylan Pharmaceuticals Recalls Xanax Drug And Device Watch
 Valsartan Blood Pressure Drug Recall Widens
Valsartan Blood Pressure Drug Recall Widens
 Mylan And Hospira Both Recall Injected Drugs After Particulate Discovered In Vials Fiercepharma
Mylan And Hospira Both Recall Injected Drugs After Particulate Discovered In Vials Fiercepharma
 Mylan Announces Voluntary Recall Of Alprazolam Tablets
Mylan Announces Voluntary Recall Of Alprazolam Tablets
 Nationwide Recall Of Valsartan Blood Pressure Medication Expanded Daic
Nationwide Recall Of Valsartan Blood Pressure Medication Expanded Daic
 Mylan Pharmaceuticals Initiates Voluntary Nationwide Recall Of One Lot Of Alprazolam Tablets Usp C Iv 0 5 Mg Due To The Potential Of Foreign Substance
Mylan Pharmaceuticals Initiates Voluntary Nationwide Recall Of One Lot Of Alprazolam Tablets Usp C Iv 0 5 Mg Due To The Potential Of Foreign Substance
 Recall Mylan Pharmaceuticals Says Trace Elements Of Ndma Found In Some Antacids Fox 59
Recall Mylan Pharmaceuticals Says Trace Elements Of Ndma Found In Some Antacids Fox 59

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.