62 total cases were reported at a rate of 45 per million doses comparable to the rate of anaphylaxis with other common vaccines. Published latest ADR adverse drug reaction data covering the period up to 14 February 2021.
 An Mrna Vaccine Against Sars Cov 2 Preliminary Report Nejm
An Mrna Vaccine Against Sars Cov 2 Preliminary Report Nejm
We conclude that the overall frequency of symptoms in both groups was low.

Vaccine adverse reaction rates. The CDC said that through Jan. For both vaccines this equates to 1 in every 333 people suffering an adverse reaction. Falsey et al 2009.
We commonly know these as side effects. Systemic adverse events among persons aged 65 years were more frequent after vaccination with a vaccine containing a high dose of 180 mcg of HA antigen 36 per 100 vaccinees compared with a standard dose of 45 mcg 24 per 100 vaccinees. Materials and Learning Tools.
Systemic reactions includes fever 103º394 ºC occurs in about 5 to 15 of vaccine recipients between the 7th to 12th day after vaccination and lasts approximately 1 to 2 days. Any side effects reported are suspected by the reporter to be caused by the vaccine. Report an Adverse Event.
This rate could actually. 18 there were 50 cases of anaphylaxis reported in recipients of the vaccine. Adverse events following immunisation AEFI for the COVID-19 vaccine are reported to the Centre for Adverse Reactions Monitoring CARM.
The most frequent adverse reaction after vaccination was soreness at the injection site in 334 followed by skin redness in 181 myalgia in 177 fatigue in 17 and febrile sensation in 152. However the absolute risk of a flu-like illness was 55 higher during the first week following influenza vaccination when compared with the third week after the injection. Typically reactions were mild and transient resolving within 3 days in the majority of subjects.
18 Zeilen The most common adverse effects are erythema 36 pain or. 4400 adverse events reported after receiving Pfizer-BioNTech vaccine Nearly 4400 adverse events were reported after people received the Pfizer-BioNTech Covid-19 vaccine in the US with 21 cases. THURSDAY March 11 2021 HealthDay News Severe reactions consistent with anaphylaxis have occurred among 247 per 10000 health care workers receiving COVID-19 vaccinations according to a research letter published online March 8 in the Journal of the American Medical Association.
But as predicted with the rise in vaccines administered came a rise in adverse reactions with 49472 reported reactions to the Pfizer vaccine and 21032 reactions to the Oxford Astrazeneca vaccine. Guide to Interpreting Data. Generally reported non-serious adverse events are consistent with information provided in the vaccine product pages.
Higher rates of non-serious adverse event reports are observed for each vaccine with serious report rates continuing to remain low. Anaphylaxis a vaccine reaction that garnered wide media coverage was found to be uncommon. After vaccination such adverse reactions began within 24 h in 706 of subjects.
That implies a rate of about 5. In some cases the fever may be coincidental due to other infections. More than 2300 Johnson Johnson vaccines were administered at the clinic on.
It doesnt always mean the vaccine did cause the side effect. These symptoms did not result in a decreased abilit. Wake County spokeswoman Stacy Beard said 18 people had an adverse reaction to the vaccine at the PNC Arena clinic.
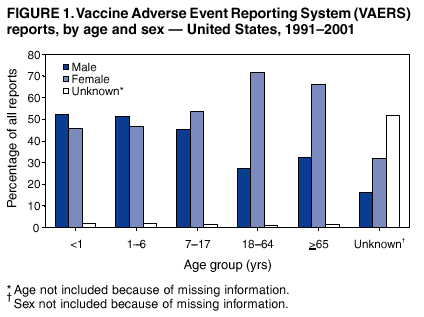 Surveillance For Safety After Immunization Vaccine Adverse Event Reporting System Vaers United States 1991 2001
Surveillance For Safety After Immunization Vaccine Adverse Event Reporting System Vaers United States 1991 2001
 The Rate Of Adverse Reactions Adrs From Cervarix Compared To That Of Download Scientific Diagram
The Rate Of Adverse Reactions Adrs From Cervarix Compared To That Of Download Scientific Diagram
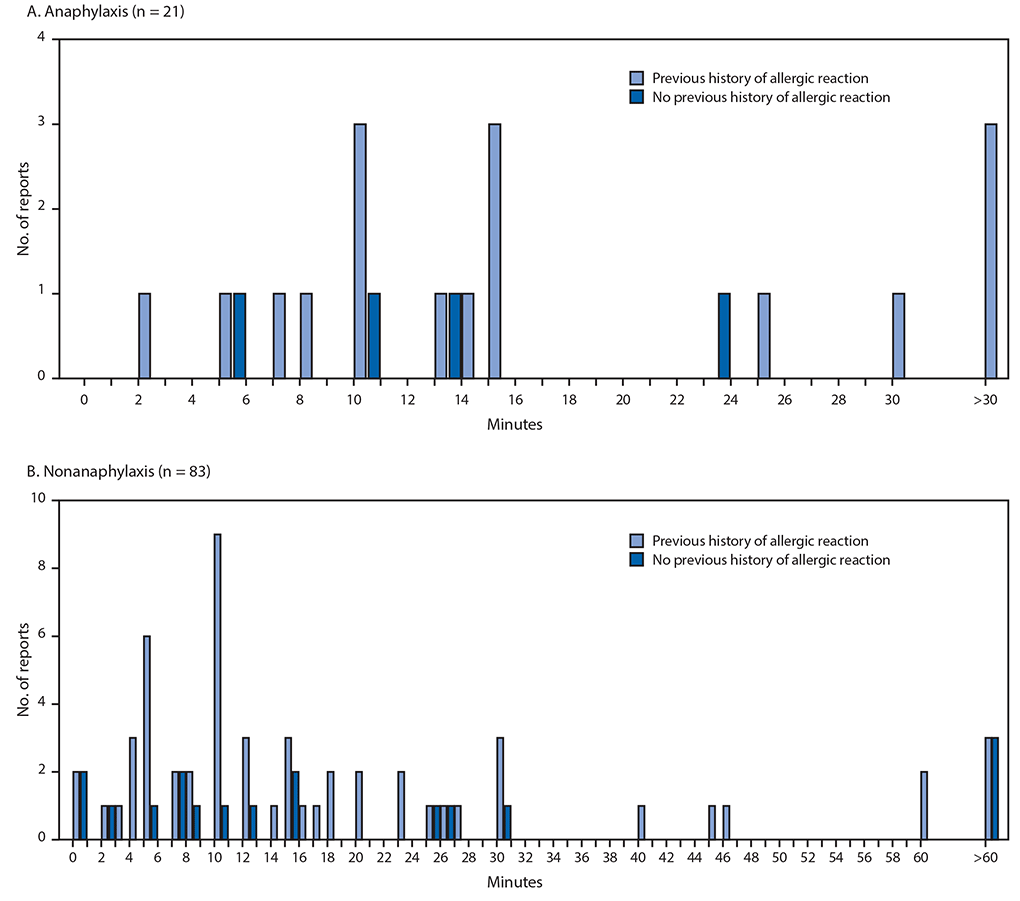 Allergic Reactions Including Anaphylaxis After Receipt Of The First Dose Of Pfizer Biontech Covid 19 Vaccine United States December 14 23 2020 Mmwr
Allergic Reactions Including Anaphylaxis After Receipt Of The First Dose Of Pfizer Biontech Covid 19 Vaccine United States December 14 23 2020 Mmwr
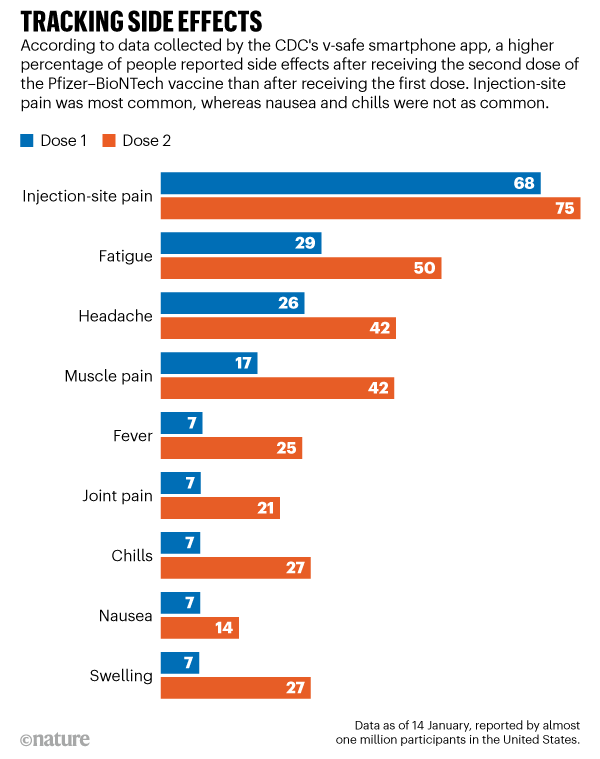 Covid Vaccines And Safety What The Research Says
Covid Vaccines And Safety What The Research Says
 Numbers And Rates Of Adverse Events Reported By Vaccination Group And Download Scientific Diagram
Numbers And Rates Of Adverse Events Reported By Vaccination Group And Download Scientific Diagram
 Vaccine Adverse Events Separating Myth From Reality American Family Physician
Vaccine Adverse Events Separating Myth From Reality American Family Physician
 Vaccine Side Effects And Sars Cov 2 Infection After Vaccination In Users Of The Covid Symptom Study App In The Uk A Prospective Observational Study The Lancet Infectious Diseases
Vaccine Side Effects And Sars Cov 2 Infection After Vaccination In Users Of The Covid Symptom Study App In The Uk A Prospective Observational Study The Lancet Infectious Diseases
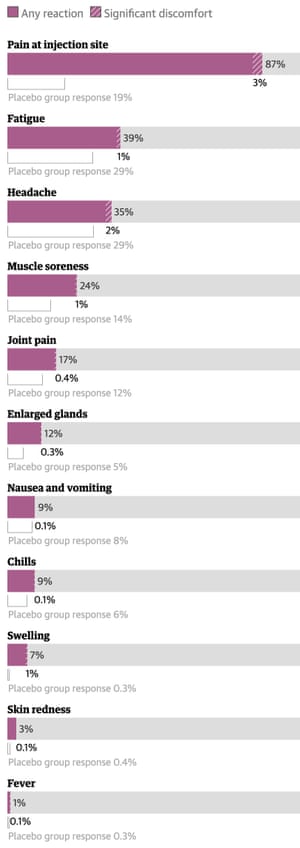 Covid Vaccine Side Effects What To Know And Why You Shouldn T Worry World News The Guardian
Covid Vaccine Side Effects What To Know And Why You Shouldn T Worry World News The Guardian
 Safety Of Influenza A H1n1 Vaccine In Postmarketing Surveillance In China Nejm
Safety Of Influenza A H1n1 Vaccine In Postmarketing Surveillance In China Nejm
 Vaccine Adverse Events Separating Myth From Reality American Family Physician
Vaccine Adverse Events Separating Myth From Reality American Family Physician
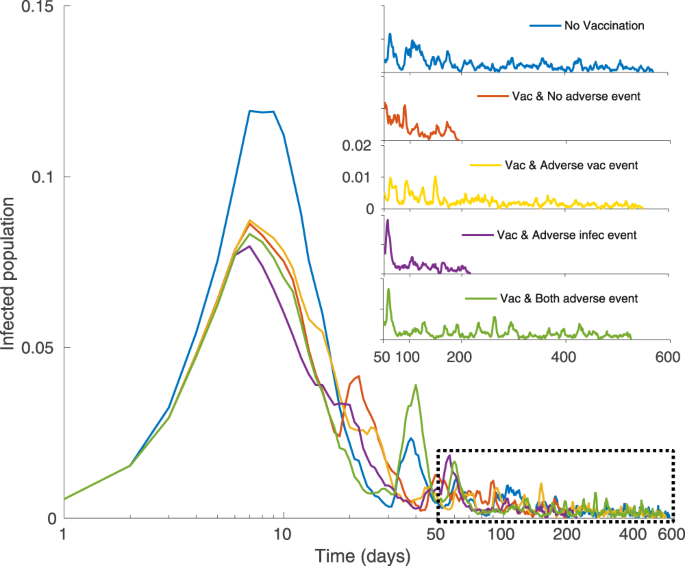 The Impact Of Rare But Severe Vaccine Adverse Events On Behaviour Disease Dynamics A Network Model Scientific Reports
The Impact Of Rare But Severe Vaccine Adverse Events On Behaviour Disease Dynamics A Network Model Scientific Reports
 Vaccine Side Effects And Sars Cov 2 Infection After Vaccination In Users Of The Covid Symptom Study App In The Uk A Prospective Observational Study The Lancet Infectious Diseases
Vaccine Side Effects And Sars Cov 2 Infection After Vaccination In Users Of The Covid Symptom Study App In The Uk A Prospective Observational Study The Lancet Infectious Diseases
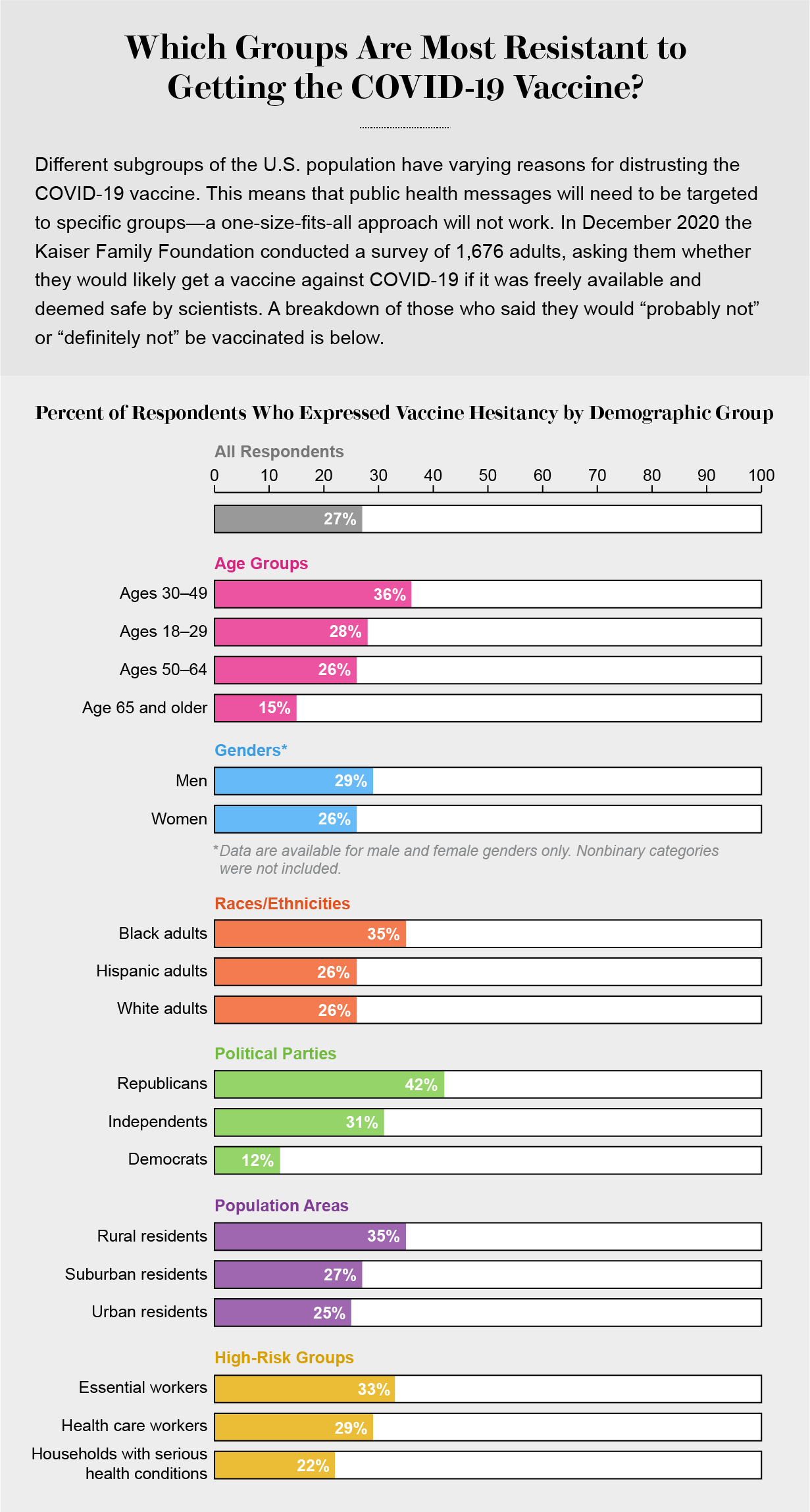 The Best Evidence For How To Overcome Covid Vaccine Fears Scientific American
The Best Evidence For How To Overcome Covid Vaccine Fears Scientific American
 Module 3 Rates Of Adverse Vaccine Reactions Who Vaccine Safety Basics
Module 3 Rates Of Adverse Vaccine Reactions Who Vaccine Safety Basics

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.